Formation & Use of Amorphous Silica

This was a waste water treatment research project of the Graz University of Technology (TU Graz) at the Institute of Applied Geosciences. The aim was to create a proper material which has a high ion exchange capacity to remove e.g. heavy metals from aqueous solutions. Through hydrothermal alteration experiments coupled with chemical modelling enabled refining selective material properties. We successfully created nanosized spherical particles (NSP) with a significant increase of metal removal capacities. The early and final results can be found in "Tailoring of hydrothermally altered diatomite for the removal of metal ions from waste water" (Raab, 2012) and in "Synthesis of hierarchically structured material: microporous diatoms and nanoporous hydroxyalumino-silicate" (Höllen et al., 2016). The following paragraphs are excerpts of those publications.


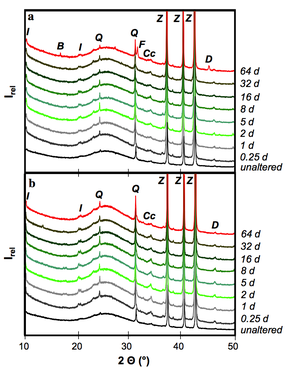
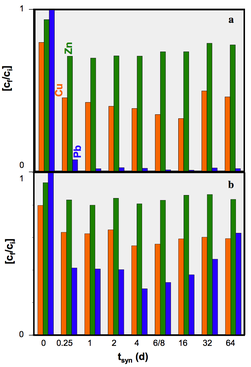
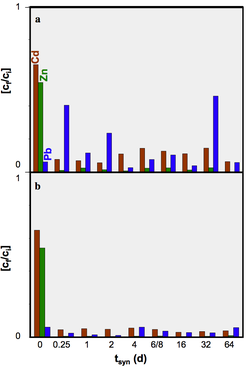
We concluded that hydrothermal alteration of naturally occurring diatomite in alkaline solution can lead to the formation of crystalline aluminosilcates. Comparingly low molarity of the alkaline solutions used in our hydrothermal alteration of diatomite suppresses the formation of crystalline aluminosilcates. The obtained NSP are partly nano-crystalline and nano-porous and seem to be formed directly in the microporous skeleton of the diatoms before their complete dissolution. The tailored material enables the application of simultaneous removal of suspended and dissolved contaminants (e.g. wastewater treatment). The challenging issue for ongoing and future studies comprise of the increase in the relative percentage of the NSP in the hierarchically structured material.
All rights reserved @ Gerald Raab






